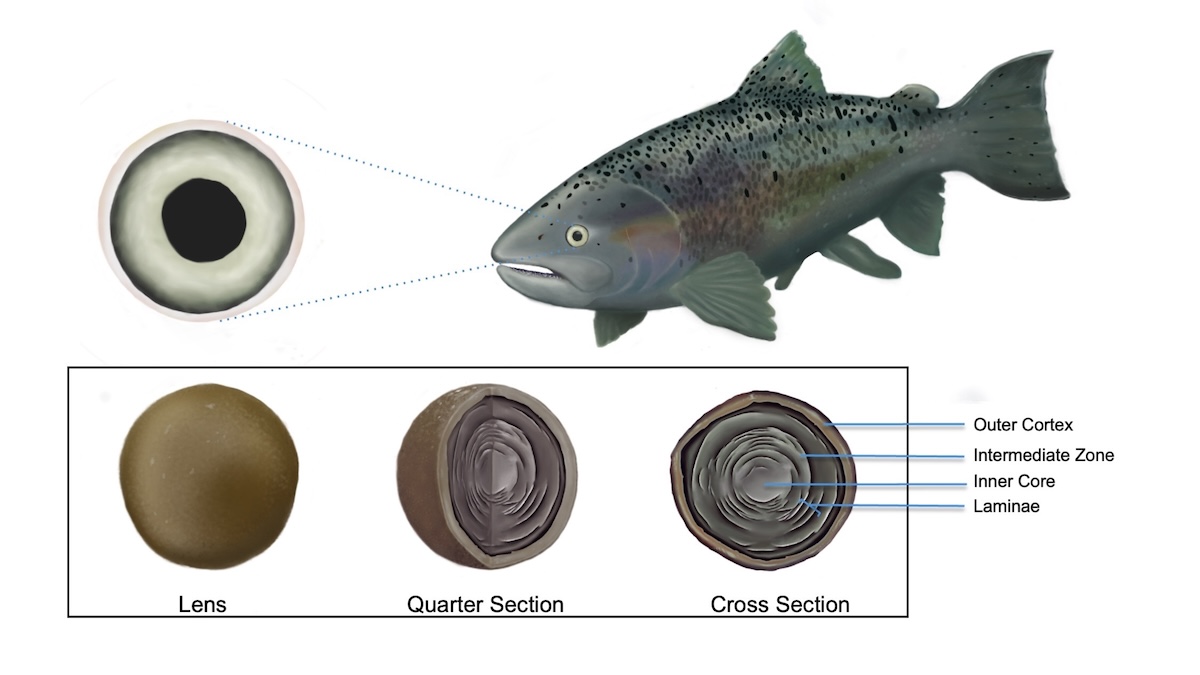
Latest
-
Getting Our Feet Wet: Bringing Photography Students into the Yolo Bypass
By Eliza Gregory . . . The first time I heard the phrase “flood-based ecosystem,” I was in New South Wales, and I was confused. I was on a 4000 km drive around the Murray Darling Basin, the largest watershed in Australia. I was with a group from Engineers Without Borders Australia, who luckily had an expansive idea of who would be fun to have along (shout out to Claire…